Abstract
Introduction:
Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 Chimeric Antigen Receptor (CAR) T-cell therapy that induces durable responses in patients with relapsed or refractory large B-cell lymphoma. At a median of 27.1 months follow-up on the ZUMA-1 trial, median overall survival (OS) was 25.8 months with 39% progression free survival (PFS) at 2 years post-infusion (Locke, Lancet Onc 2019). We previously reported outcomes of axi-cel patients treated with standard of care therapy at a median follow up of 12.9 months, including 42% who did not meet eligibility criteria for ZUMA-1 based on co-morbidities (Nastoupil, JCO 2020). Here we report results from this cohort at a median follow up of 32.4 months, as well as late outcomes of interest including cytopenias, infections and secondary malignancies.
Methods and Results:
The US Lymphoma CAR-T Consortium comprised of 17 US academic centers who contributed data independent of the manufacturer. Two hundred and ninety-eight patients underwent leukapheresis with intent to manufacture standard of care axi-cel as of September 30, 2018. In infused patients (n=275), OS and PFS were calculated from date of infusion.
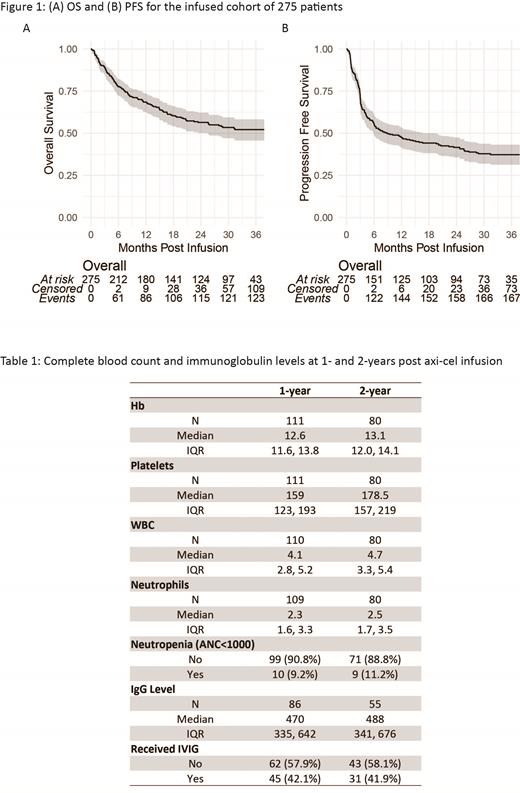
After median follow-up of 32.4 months (95% CI 31.1 - 34.3), median OS was not reached (95% CI 25.6 - not evaluable) (Figure 1A) with 1-, 2- and 3-year OS of 68.5% (95% CI 62.6-73.7), 56.4% (95% CI 50.1-62.2) and 52.2% (95% CI 45.7-58.2%), respectively. Median PFS was 9 months (95% CI 5.9-19.6) (Figure 1B); 1-, 2- and 3-year PFS was 47.4% (95% CI 41.4-53.2), 41.6% (95% CI 35.6-47.5) and 37.3% (95% CI 31.3-43.2), respectively. Twenty-seven PFS events occurred at or after 1 year post infusion;19 events were progressive lymphoma, with the latest relapse observed 28 months after axi-cel infusion. Eight patients died while in remission from their lymphoma: 4 from secondary malignancy, 3 from infection, and 1 from unknown causes. Results of multivariable modeling were similar to our prior analysis: factors associated with both a shorter PFS and shorter OS included male sex, elevated pre-lymphodepletion LDH, and poor ECOG status.
Complete blood count and B- and T-cell recovery data were collected at 1 and 2-years post-infusion, excluding patients who had relapsed or been treated for secondary malignancy at time of collection (Table 1). Rates of neutropenia (absolute neutrophil count ≤1000) at 1- and 2- years were 9.2% (10/109) and 11.2% (9/80) and rates of CD4 count ≤200/ul were 62% (23/37) and 27% (7/26). Recovery of B cells was seen in 54% (15/28) and 57% (13/23) at 1-and 2-years post infusion.
Infections were reported in 31.2% (34/109) patients between 6- and 12-months post infusion, and 17% (18/109) were severe, requiring either hospitalization and/or IV antibiotics. Twenty-one patients (24%, 21/89) had an infection between 1- and 2- years, 11% of which were severe. Twenty percent (10/49) of patients between 2- and 3-years had an infection and 4 (8%) were severe. Neutropenia, low CD4 counts, and IgG levels were not associated with infection, though patients with infection between 6-12 months were more likely to have received IVIG (p<0.001). No patient in this cohort died of COVID-19.
Twenty-two of 275 (8%) patients were diagnosed with subsequent malignancy after axi-cel treatment: 14/275 (5%) patients were diagnosed with myeloid malignancies (MDS (n=12), AML (n=1), CMML (n=1)); other malignancies included squamous cell carcinoma of skin (n=3); sarcoma (n=1); endometrial (n=1); lung (n=1); mesothelioma (n=1) and AITL (n=1). Patients with myeloid malignancy had a median age of 62 at axi-cel apheresis (IQR 56-67), 64% were male and median lines of prior therapy was 4 (IQR 3-6), including 36% with a prior autologous stem cell transplant. Eleven patients were in remission from lymphoma at myeloid malignancy diagnosis, while 3 were diagnosed after progression and interval therapy.
Conclusion:
This multi-center retrospective study showed similar long-term results to the ZUMA-1 trial, despite including patients who did not meet ZUMA-1 eligibility criteria based on comorbidities. Sixteen percent of PFS events were seen after 1 year, largely due to disease progression. Late infection was common but was not explained by persistent neutropenia or low CD4 counts. Subsequent malignancy, including MDS, occurred in 8% of patients and require further study to better identify patients at risk.
J.Y.S and M.D.J contributed equally; S.D and M.L contributed equally.
Jain: BMS, Kite/Gilead, Novartis, Precision Biosciences, Takeda: Consultancy. Nastoupil: Pfizer: Honoraria, Research Funding; MorphoSys: Honoraria; Takeda: Honoraria, Other: DSMC, Research Funding; ADC Therapeutics: Honoraria; IGM Biosciences: Research Funding; Genentech: Honoraria, Research Funding; Epizyme: Honoraria, Research Funding; Caribou Biosciences: Research Funding; Gilead/Kite: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding; Denovo Pharma: Other: DSMC; Bayer: Honoraria; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding. Ghobadi: Atara: Consultancy; Amgen: Consultancy, Research Funding; Wugen: Consultancy; Celgene: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding. Lin: Gamida Cell: Consultancy; Juno: Consultancy; Merck: Research Funding; Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Janssen: Consultancy, Research Funding; Sorrento: Consultancy; Legend: Consultancy; Takeda: Research Funding; Vineti: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Reagan: Seagen: Research Funding; Kite, a Gilead Company: Consultancy; Genentech: Research Funding; Curis: Consultancy. Oluwole: Curio Science: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. McGuirk: EcoR1 Capital: Consultancy; Gamida Cell: Research Funding; Bellicum Pharmaceuticals: Research Funding; Novartis: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Novartis: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Astelllas Pharma: Research Funding; Pluristem Therapeutics: Research Funding; Fresenius Biotech: Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding. Deol: Kite, a Gilead Company: Consultancy. Sehgal: Juno/Celgene: Research Funding; Kite/Gilead: Research Funding. Goy: AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; LLC(Targeted Oncology): Consultancy; Xcenda: Consultancy, Honoraria; Xcenda: Consultancy; Acerta: Consultancy, Research Funding; AbbVie/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Elsevier's Practice Update Oncology, Intellisphere, LLC(Targeted Oncology): Consultancy; Hoffman la Roche: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie/Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Phamacyclics: Research Funding; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Michael J Hennessey Associates INC: Consultancy; Genentech/Hoffman la Roche: Research Funding; MorphoSys: Honoraria, Other; Karyopharm: Research Funding; Vincerx pharma: Membership on an entity's Board of Directors or advisory committees; Physicians' Education Resource: Consultancy, Other: Meeting/travel support; Incyte: Honoraria; Medscape: Consultancy; Novartis: Consultancy, Honoraria; Genomic Testing Cooperative: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Infinity/Verastem: Research Funding; Rosewell Park: Consultancy; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; OncLive Peer Exchange: Honoraria; Elsevier PracticeUpdate: Oncology: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Constellation: Research Funding; COTA (Cancer Outcome Tracking Analysis): Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Hackensack Meridian Health, Regional Cancer Care Associates/OMI: Current Employment. Hill: Pfizer: Consultancy, Honoraria; Beigene: Consultancy, Honoraria, Research Funding; Gentenech: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Incyte/Morphysis: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Celgene (BMS): Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Andreadis: CRISPR Therapeutics: Research Funding; GenMAB: Research Funding; Novartis: Research Funding; Roche: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Epizyme: Honoraria; Incyte: Honoraria; TG Therapeutics: Honoraria; Kite: Honoraria; Karyopharm: Honoraria; Atara: Consultancy, Honoraria; BMS: Research Funding; Merck: Research Funding. Muñoz: Seagen: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Kite, a Gilead Company: Consultancy, Speakers Bureau; Pfizer: Consultancy; Janssen: Consultancy, Speakers Bureau; Juno/Celgene: Consultancy; Bristol Myers Squibb: Consultancy; Alexion: Consultancy; BeiGene: Consultancy, Speakers Bureau; Fosunkite: Consultancy; Innovent: Consultancy; Debiopharma: Consultancy; Karyopharm: Consultancy; Genmab: Consultancy; ADC Therapeutics: Consultancy; Epizyme: Consultancy; Servier: Consultancy; Acrotech: Speakers Bureau; Verastem: Speakers Bureau; AstraZeneca: Speakers Bureau; Celgene/Bristol Myers Squibb: Speakers Bureau; Genentech: Speakers Bureau; Aurobindo: Speakers Bureau; Physicians' Education Resource: Honoraria; Kyowa Kirin: Consultancy, Honoraria, Speakers Bureau; OncView: Honoraria; Targeted Oncology: Honoraria. Westin: AstraZeneca: Consultancy, Research Funding; 47 Inc: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Morphosys: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Umoja: Consultancy; Iksuda Therapeutics: Consultancy; MorphoSys: Consultancy, Research Funding; Curis: Research Funding; Genentech: Consultancy, Research Funding. Chavez: Bristol Myers Squibb: Speakers Bureau; Karyopharm Therapeutics: Consultancy; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Kite/Gilead: Consultancy; Abbvie: Consultancy; MorphoSys: Speakers Bureau; BeiGene: Speakers Bureau; Novartis: Consultancy; Adaptive: Research Funding; Epizyme: Speakers Bureau; AstraZeneca: Research Funding. Bennani: Kyowa Kirin: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Purdue Pharma: Other: Advisory Board; Verastem: Other: Advisory Board; Vividion: Consultancy, Other: Advisory Board. Vose: Kite, a Gilead Company: Honoraria, Research Funding. Miklos: Pharmacyclics: Patents & Royalties; Adaptive Biotechnologies, Novartis, Juno/Celgene-BMS, Kite, a Gilead Company, Pharmacyclics-AbbVie, Janssen, Pharmacyclics, AlloGene, Precision Bioscience, Miltenyi Biotech, Adicet, Takeda: Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company, Amgen, Atara, Wugen, Celgene, Novartis, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Bioscience, Adicet, Pharmacyclics, Janssen, Takeda, Adaptive Biotechnologies and Miltenyi Biotechnologies: Consultancy; Pharmacyclics, Amgen, Kite, a Gilead Company, Novartis, Roche, Genentech, Becton Dickinson, Isoplexis, Miltenyi, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Biosciences, Adicet, Adaptive Biotechnologies: Research Funding. Neelapu: Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees. Locke: GammaDelta Therapeutics: Consultancy, Other: Scientific Advisory Role; BMS/Celgene: Consultancy, Other: Scientific Advisory Role; Bluebird Bio: Consultancy, Other: Scientific Advisory Role; Calibr: Consultancy, Other: Scientific Advisory Role; Amgen: Consultancy, Other: Scientific Advisory Role; Allogene Therapeutics: Consultancy, Other: Scientific Advisory Role, Research Funding; Cellular Biomedicine Group: Consultancy, Other: Scientific Advisory Role; Wugen: Consultancy, Other; Takeda: Consultancy, Other; Novartis: Consultancy, Other, Research Funding; Umoja: Consultancy, Other; Cowen: Consultancy; EcoR1: Consultancy; Emerging Therapy Solutions: Consultancy; Gerson Lehrman Group: Consultancy; Moffitt Cancer Center: Patents & Royalties: field of cellular immunotherapy; Legend Biotech: Consultancy, Other; Janssen: Consultancy, Other: Scientific Advisory Role; Kite, a Gilead Company: Consultancy, Other: Scientific Advisory Role, Research Funding; Iovance Biotherapeutics: Consultancy, Other: Scientific Advisory Role. Dahiya: Kite, a Gilead Company: Consultancy; Atara Biotherapeutics: Consultancy; Miltenyi Biotech: Research Funding; Jazz Pharmaceuticals: Research Funding; BMS: Consultancy. Lunning: Spectrum: Consultancy; AstraZeneca: Consultancy; Acrotech: Consultancy; Legend: Consultancy; Novartis: Consultancy; Kyowa Kirin: Consultancy; Karyopharm: Consultancy; Morphosys: Consultancy; TG Therapeutics: Consultancy; Janssen: Consultancy; AbbVie: Consultancy; Verastem: Consultancy; Myeloid Therapeutics: Consultancy; Celgene, a Bristol Myers Squibb Co.: Consultancy; Daiichi-Sankyo: Consultancy; Beigene: Consultancy; ADC Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal